How Well are COVID Vaccine Medium/Long-Term Risks Studied? UPDATE: CDC Data
re: ethics in medicine
re: COVID-19
re: Lies, Damn Lies, and Statistics: VAERS Reporting for COVID Vaccine Adverse Events vs COVID Deaths
If you don’t look for something, you are not going to find it. And if you look in the wrong places, you won’t find it either. Obvious? Not to vaccine makers and the FDA/CDC.
When you look only for benefits, and you look only for simplistic endpoints (antibody levels, immediate and obvious adverse reactions), it’s like blind people feeling up a small part of the elephant.
I’m asking for what any reasonable and ethical and responsible person would ask for: show credible evidence of medium/long-term safety, based on well-known biomarkers for disease before and after vaccination. Get a baseline and then compare things a day/week/month/quarter/year out. Get some real world data, and do it for at least 10K people. I also want independently obtained and evaluated and publicly available data because of the conflicts of interest involved (vaccine makers, FDA, CDC are massively conflicted).
Am I wrong to expect such data as a minimum credible standard, in order to have confidence in vaccine safety? Or are we all just human guinea pigs, with children next?
Note that I am not taking a position on whether ill effects occur in the medium/long term, but simply asking whether the safety science is credible. Because it looks non-credible to me.
So I make the same challenge here as with other recent posts: can an MD or epidemiologist or similar with appropriate skills or a data scientist explain how the safety studies then or now (if they even exist!) meet credible safety science?
NIH.gov: Why are we vaccinating children against COVID-19?
2021-09-14, Ronald N. Kostoff a, *, Daniela Calina b, Darja Kanduc c, Michael B. Briggs d, Panayiotis Vlachoyiannopoulos e, Andrey A. Svistunov f, Aristidis Tsatsakis
Emphasis added.
...In Pfizer’s proposed clinical trials for the mRNA “vaccine” (Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals - https://clinicaltrials.gov/ct2/show/NCT04368728), the focus was on determining 1) adverse events/symptoms, 2) SARS-CoV-2 serum neutralizing antibody levels, 3) SARS-CoV-2 anti-S1 binding antibody levels and anti- RBD binding antibody levels, and 4) effectiveness. These metrics are all related to safety at the symptom level and performance.
However, symptoms/diseases are typically end points of processes that can take months, years, or decades to surface. During that symptom/disease development period, many biomarker early warning indicators tend to exhibit increasing abnormalities that reflect an increasing predisposition to the eventual symptom/disease. Thus, serious symptoms/diseases that ordinarily take long periods to develop would be expected to be rare events if they occurred shortly following an inoculation. If the clinical trials that were performed by Pfizer and Moderna were designed to focus on efficacy and only adverse effects at the symptom level of description as an indicator of safety, the trial results would be limited to the identification of rare events, and the trial results would potentially under-estimate the actual pre-symptom level damage from the inoculations.
Credible safety science applied to this experiment would have required a much more expansive approach to determining effects on a wide variety of state and flux metrics that could serve as early warning indicators of potentially serious symptoms/disease, and might occur with much higher frequencies at this early stage than the rare serious symptoms. The only mention of these other metrics in the above pro- posal is in the Phase I trial description: “Percentage of Phase 1 partici- pants with abnormal haematology and chemistry laboratory values”, to be generated seven days after dose 1 and dose 2.
A paper published in NEJM in December 2020 [34] summarized the Phase 1 results. The focus was on local and systemic adverse events and efficacy metrics (antibody responses). The only metrics other than these reported were transiently decreased lymphocyte counts.
We view this level of reporting as poor safety science for the following reasons. Before the clinical trials had started, many published articles were reporting serious effects associated with the presence of the SARS-CoV-2 virus such as hyperinflammation, hypercoagulation, hypoxia, etc. SARS-CoV-2 includes the S1 Subunit (spike protein), and it was not known how much of the damage was associated with the spike protein component of SARS-CoV-2. A credible high-quality safety science experiment would have required state measurements of specific biomarkers associated with each of these abnormal general biomarkers before and after the inoculations, such as d-dimers for evidence of enhanced coagulation/clotting; CRP for evidence of enhanced inflammation; troponins for evidence of cardiac damage; occludin and claudin for evidence of enhanced barrier permeability; blood oxygen levels for evidence of enhanced hypoxia; amyloid-beta and phosphorylated tau for evidence of increased predisposition to Alzheimer’s disease; Serum HMGB1, CXCL13, Dickkopf-1 for evidence of an increased disposition to autoimmune disease, etc.
A credible high-quality safety science experiment would have required flux measurements of products resulting from the mRNA interactions, from the LNP shell interactions, from dormant viruses that might have been stimulated by the mRNA-generated spike protein, etc., emitted through the sweat glands, faeces, saliva, exhalation, etc.
Most importantly, these types of measurements would have shown changes in the host that did not reach the symptom level of expression but raised the general level of host abnormality that could predispose the host to a higher probability of serious symptoms and diseases at some point in the future.
Instead, in the absence of high-quality safety science reflected in these experiments, all that could be determined were short-term adverse effects and deaths. This focus on symptoms masked the true costs of the mRNA intervention, which would probably include much larger numbers of people whose health could have been degraded by the intervention as evidenced by increased abnormal values of these biomarkers. For example, the trials and VAERS reported clots that resulted in serious symptoms and deaths but gave no indication of the enhanced predisposition to forming serious clots in the future with a higher base of micro-clots formed because of the mRNA intervention. The latter is particularly relevant to children, who have a long future that could be seriously affected by having an increased predisposition to multiple clot-based (and other) serious diseases resulting from these inoculations.
...
WIND: I’ve been deeply skeptical of the COVID vaccine safety profile since the initial claims, as I could find no credibility in the crude metrics applied for safety. Are we willing to harm an entire generation of children without having better safety science?
Similarly, for those who have had COVID and thus have natural immunity (including an estimated 42% of children), I am unaware of any credible safety science as per above, with all science to date suggesting more adverse reactions to the COVID vaccines for those with natural immunity—potentially a much worse risk profile with dubious benefits, unproven by science and with pathetically inadequate risk assessment.
In my opinion, anyone with natural immunity is taking a totally unknown risk to get the vaccine for no credible benefit (rare cases excepted), given the apparent absence of credible safety science.
Now let’s look at the CDC data published Oct 29, 2021.
CDC: COVID-19 Vaccination and Non–COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021
2021-10-29
Emphasis added.
What is already known about this topic?
Although deaths after COVID-19 vaccination have been reported to the Vaccine Adverse Events Reporting System, few studies have been conducted to evaluate mortality not associated with COVID-19 among vaccinated and unvaccinated groups.
What is added by this report?
During December 2020–July 2021, COVID-19 vaccine recipients had lower rates of non–COVID-19 mortality than did unvaccinated persons after adjusting for age, sex, race and ethnicity, and study site.
What are the implications for public health practice?
There is no increased risk for mortality among COVID-19 vaccine recipients. This finding reinforces the safety profile of currently approved COVID-19 vaccines in the United States. All persons aged ≥12 years should receive a COVID-19 vaccine.
...
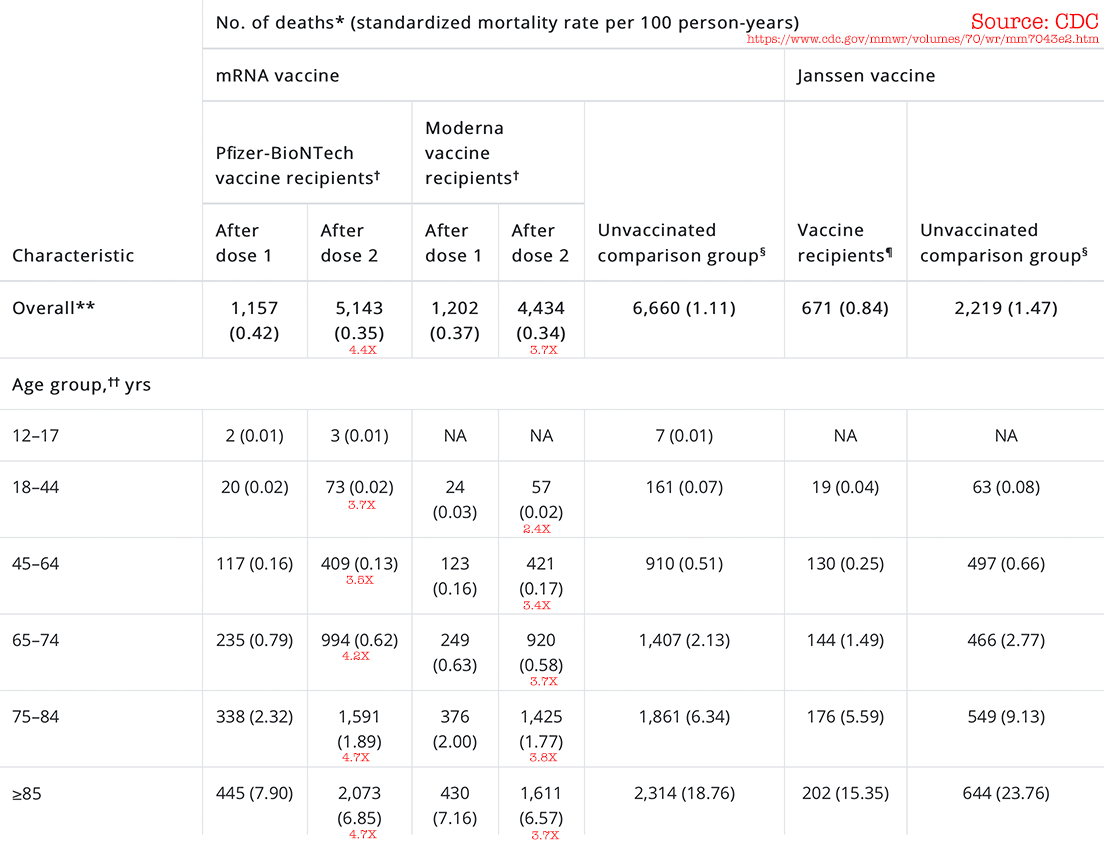 Source: https://www.cdc.gov/mmwr/volumes/70/wr/mm7043e2.htm
Source: https://www.cdc.gov/mmwr/volumes/70/wr/mm7043e2.htm
WIND: open-and-shut case as in “no increased risk for mortality among COVID-19 vaccine recipients”, right? Not really: the study doesn’t add any clarity as to whether the vaccines causes deaths (or serious and lasting AE). In medicine, such things become very important over time. And of course risk assessment weighing all the negatives against the positives is needed. The study isn’t doing that balancing, so it comes across as one-sided to me. But maybe that’s all they can do right now.
See also: A Review and Autopsy of Two COVID Immunity Studies. The CDC has little credibility these days when they keep producing crap studies to support the narrative, rather than doing real science.
Observational studies can be scientific swiss cheese. To be perhaps too skeptical:
First, it appears to undermine its own findings by showing that the vaccinated group has far lower mortality from non-COVID situations. That’s possible if the vaccine kept people healthier (stronger), as in reducing vulnerability to death from other causes after a COVID infection. It’s also possible that the non-random nature of the study has a built-in bias.
Second, the massive increase in deaths with the 2nd dose of the Moderna and Pfizer vaccines is not investigated. Maybe this study’s job was not to do so, but it should be addressed. And maybe it is just a longer timeline, so higher deaths. But to know that, we need deaths on a timeline.
Third, short of death (a very adverse event), adverse events are not considered. Permanent disability is a real thing and it would be good to see that studied (vaccination vs infection outcomes). The aRR considers only deaths, but there are many terrible diseases (e.g., neurological conditions) that I’d be loathe to suffer from.
Huge increase in post-2nd-dose deaths
The CDC’s data appears to support the assertions in the paper referenced earlier.
The large post-2nd-dose death surge needs analysis and explanation, along with supporting data showing exactly the kind of graph shown earlier—the timeline of those post-2nd-dose deaths (ditto for 1st-dose deaths). Regrettably the study provides no insight into those deaths.
Given that the 2nd dose is typically given ~2 weeks after the first dose, the death count after the first dose covers a limited time period, with the 2nd dose covering a much longer time period. That might be all there is too it. But the absence of such data and analysis of it leaves the study open to concerns about those deaths—caused by the vaccine or not? I’d like to see a timeline vs baseline deaths such as in the paper above.